Explain the Difference Between Exothermic and Endothermic
Thermic refers to heat just as in the word thermometer Exo means outside and endo means inside EXOTHERMIC REACTION. In thermodynamics these two types of reactions are classified as exothermic or endothermic respectively.
Difference Between Endothermic And Exothermic Reactions With Comparison Chart Bio Differences
Difference between Endothermic and Exothermic Reaction.

. When a reaction proceeds it either releases energy to or absorbs energy from its surroundings. An endothermic reaction feels cold to the touch. An exothermic reaction expels heat.
This is possible due to cohesive forces. 1 Explain the difference between an EXOTHERMIC reaction and an ENDOTHERMIC reaction. An endothermic reaction has a positive ΔH and absorbs heat from the surroundings.
Endothermic reactions If forming new bonds in the products releases. Endothermic is a reaction that need to be supplied with energy. An exothermic reaction has an overall negative enthalpy change and releases heat to its question_answer.
Our mission is to provide a free world-class education to anyone anywhere. 5 rows In simple terms the endothermic reactions absorb energy from the surrounding that is in the form. Reactions that absorb energy from their surroundings resulting in a net increase in energy and q are called endothermic.
Exothermic Reactions Contain Less Energy. In endothermic processes reactants possess lower potential energy than the product. A look at a seductive but wrong Gibbs spontaneity proof.
Exothermic take place when two substances are mixed together and. Its submitted by dispensation in the best field. The difference between endothermic and exothermic energy is that exothermic energy is the reaction that releases energy and endothermic is the.
Endothermic is the exact opposite. An exothermic reaction has a negative enthalpy difference. 5 rows The major difference between endothermic and exothermic reactions as their names suggest is.
The digestion of living beings. Examples of exothermic reactions are. Exothermic Reactions Make Surroundings Hotter.
Show your understanding using an energy potential diagram. The difference between esothermic and endothermic. A chemical reaction that releases energy usually in the form of heat.
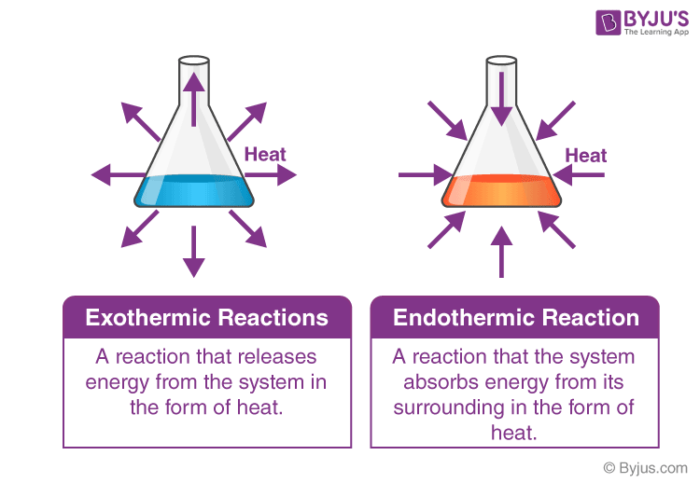
The main difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding whereas exothermic reactions release energy to the surrounding. An easy way to remember the difference between these two reaction types is by their prefixes. The key difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding environment whereas exothermic reactions release energy to the surrounding environment.
An exothermic reaction releases energy and feels warm while an endothermic reaction. The main difference is that exothermal reactions release energy in the surroundings while an endothermic reaction absorbs energy from the surroundings. Explain the difference between an exothermic and an endothermic reaction.
Burn coal wood fuel plastic among others. We allow this kind of Endothermic Reaction graphic could possibly be the most trending topic bearing in mind we allowance it in google pro or facebook. The endothermic reactions are when the system takes up the energy in the form of light or heat.
Reactions in which there is a release of energy to the surroundings resulting in a net decrease and q are called. During this process. The oxidation of metals.
This is the currently selected item. 5 rows Photosynthesis is a popular example of an endothermic chemical reaction. 14 Exothermic reactions give off energy and usually have stronger products than reactants.
15 Surface tension is property that allows liquids to repel or resist external forces. Remember to explain the difference using scientific terms such as. An exothermic reaction feels warm to the touch.
3 marks 2 Explain how a catalyst changes the rate of a reaction. In contrast exothermic systems give up heat or light energy as the reaction proceeds. Khan Academy is a 501c3 nonprofit organization.
An exothermic reaction creates heat whereas a endothermic reaction absorbs heat. Explain the difference between reactions that increase in energy and those that decrease in energy. Enthalpy energy reactant products.
An exothermic reaction has a negative ΔH and gives off heat to the surroundings. We identified it from well-behaved source. Here are a number of highest rated Endothermic Reaction pictures upon internet.
Once started exothermic reactions tend to keep going as each reaction releases more energy to fuel neighboring molecules. It can change into other forms such as heat sound light etc. The difference between endothermic and exothermic reactions lies in the words themselves.
An endothermic reaction absorbs the energy of its surroundings. Energy is the capacity to do work. Thus in order to react they absorb the energy from the environment.
Endo- means to draw in and exo- means to give off. Each and every chemical reaction can be grouped into these two categories by calculating the enthalpy change in the reaction. In a system energy can do work.
Conclusion this esothermic and endothermic reactions can be classified on the basis of the transfer of energy between the system and the surrounding environment. The final products are stable in exothermic reactions. Donate or volunteer today.

7 Difference Between Exothermic And Endothermic Reaction With Examples Viva Differences

Introduction Of Difference Between Exothermic And Endothermic Reactions In Chemistry Aesl
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples

Difference Between Exothermic And Endothermic Energy Starting At 1 48 6th Grade Science Physical Science Exothermic Reaction
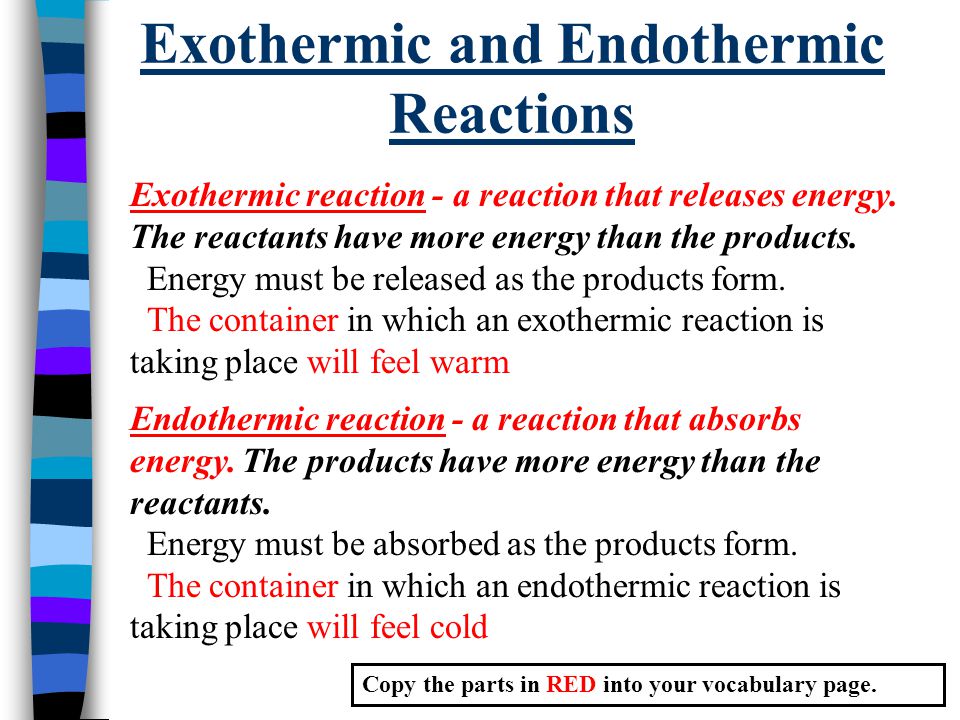
Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up Name 2 Ways You Could Speed Up A Chemical Reaction Ppt Video Online Download

Endothermic Vs Exothermic Teaching Chemistry Chemistry Worksheets Science Teaching Resources
Solved What Is The Biggest Difference Between An Exothermic And Course Hero
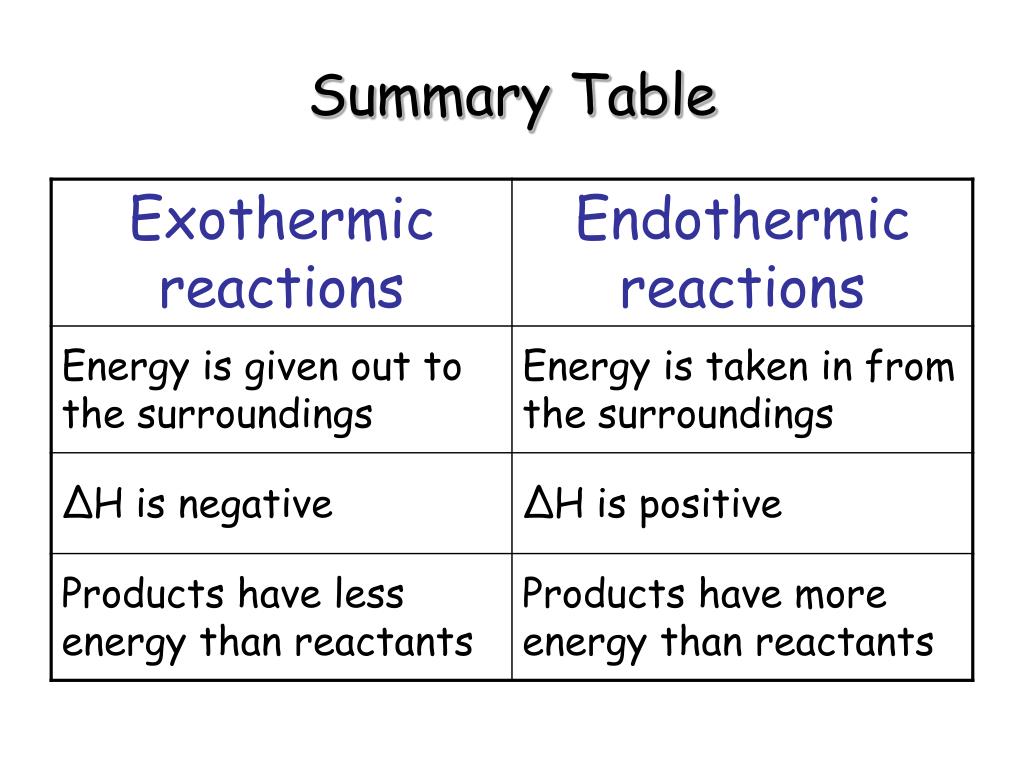
Ppt Exothermic And Endothermic Reactions Powerpoint Presentation Free Download Id 4499042

What Is The Difference Between Endothermic And Exothermic Edurev Neet Question
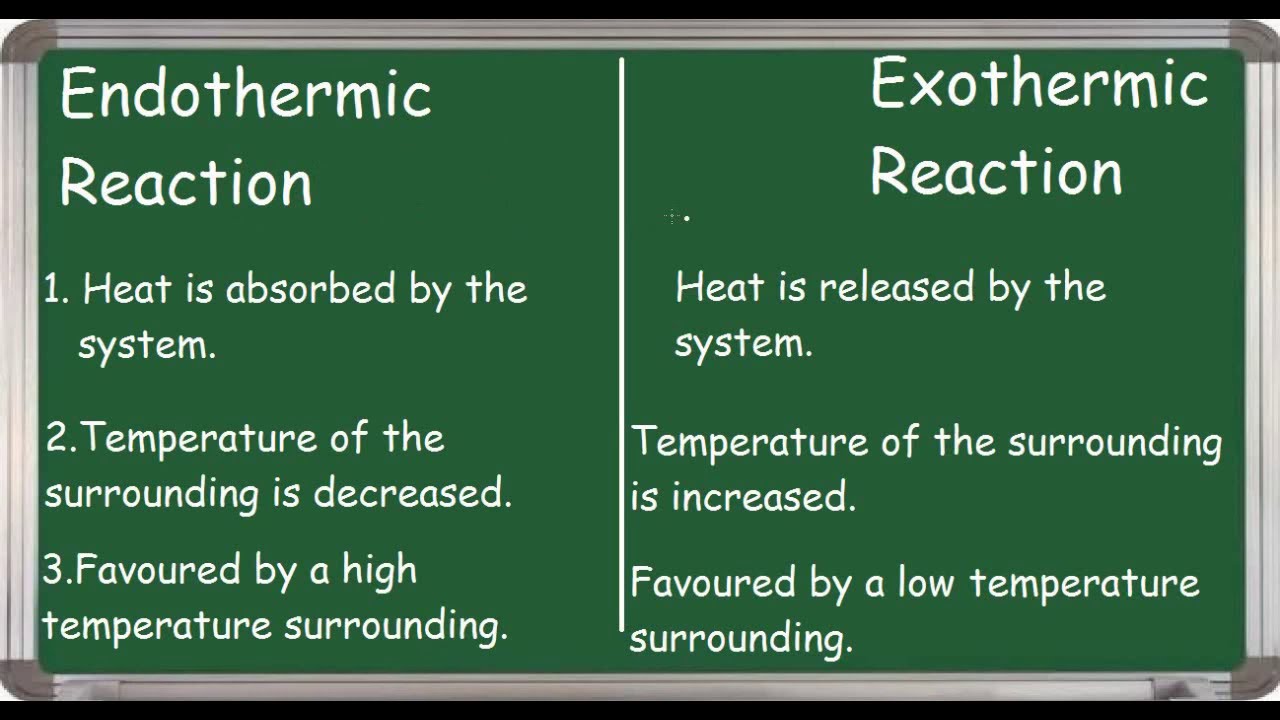
Endothermic Vs Exothermic Reaction Differences Youtube
Difference Between Endothermic And Exothermic Reactions Chemistry

Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up An Increase In Temperature Makes A Reaction Speed Ppt Download

Endothermic And Exothermic Reactions Lab Iteachly Com

Introduction Of Difference Between Exothermic And Endothermic Reactions In Chemistry Aesl

Difference Between Endothermic And Exothermic Reaction Brainly In

Exothermic And Endothermic Reactions Definition Examples And Differences

Distinguish Between Endothermic Reaction And Exothermic Reaction


Comments
Post a Comment